Enantioselective organocatalyst, the use of small organic molecules to enhance the rate of the reactions and to promote enantioselectivity, is a methodology which has recently reborn and attracted a lot of interest.
The synthesis of novel organocatalysts and their use in old or novel synthetic transformations is of paramount importance, because the needing of enantiopure substances has increased in medicinal, biological and material applications. In this way, our research group are mainly interested in three different, although related, topics:
- Synthesis of novel organocatalysts
- Supporting of small chiral molecules on polymers to be used as easily recoverable organocatalysts.
- Searching for novel applications of organocatalyts in enantioselective transformations leading to targets of synthetic or biological interest.
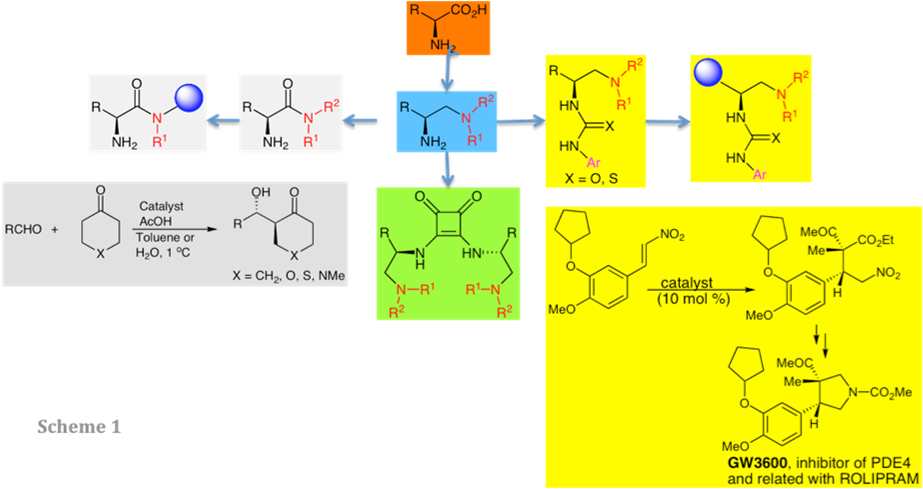
The first point has been developed by preparing a series of catalysts, in a modular way, taking into account three main points: a) The starting material would be easily accessible and cheap; b) both enantiomers of the catalysts must be accessible, and c) the synthesis should be able to provide the ability to fine-tune the substituents at both the stereocenter and the nitrogen atom. Following these points the natural a-amino acids fulfill all these conditions and they were the starting material of choice for the preparation of two different types of catalysts.
The first ones are amides to be used as bifunctional organocatalysts in reactions that occur throughout enamines or iminium intermediates.
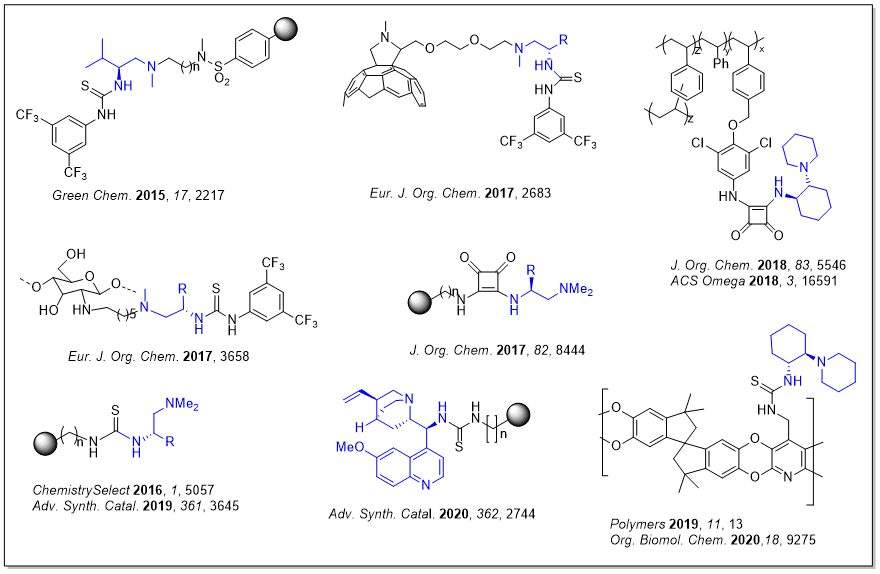
A second group corresponds to bifunctional organocatalysts that are able to activate both the nucleophilic and the electrophilic reactants (ureas, thioureas and squaramides).
Some of those catalysts have been anchored on different polymeric materials such as chlorosulphonyl polystyrene, aminoethyl polystyrene, Polymers of Intrinsic Microporosity (PIMs), chitosan, etc. In a different approach, some of these catalysts have been bottom-up synthesized by copolymerization of functionalized monomers.
Scheme 2
These catalysts are able to promote a series of enantioselective transformations such as inter and intramolecular aldol reactions, Michael additions, anhydride desymmetryzations, additions to imines (aza-Henry, aza-Friedel Crafts and Mannich reactions), etc… The catalysts are recoverable and reusable, and some of the supported catalysts has been used in a flow process allowing the synthesis of 3-amino-2-oxindole derivatives in multigram scale with high yield and enantioselectivity (Scheme 3).

Scheme 3
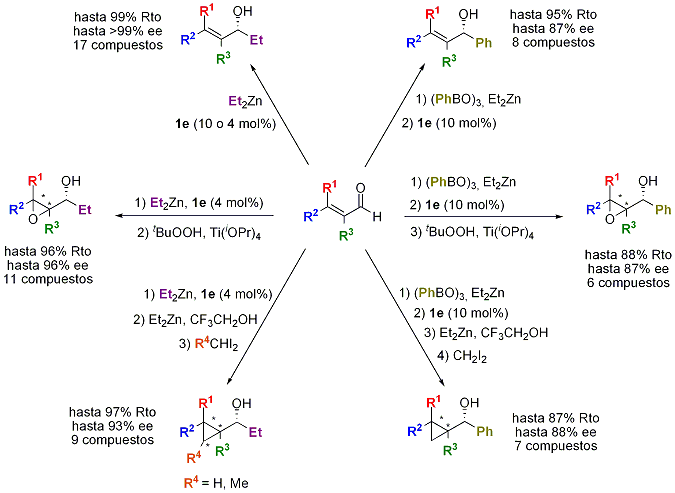
A different approach to enantioselective transformations is the addition of different nucleophiles to carbonyl derivatives catalyzed by chiral amino alcohols. In this way, hydroxy pyrrolidines with a chiral appendage derived from (-)-8-amino menthol have been identified as excellent catalysts for adition of alkyl or arylzincs to aldehydes and ketones. In addition, novel enantioselective one pot processes have been developed by sequential addition of organometallics and functionalization of the double bond in a,b-unsaturated aldehydes and ketones (Scheme 4).
Scheme 4
More recently, an efficient, enantioselective Me2Zn-mediated monoaddition of phenylacetylene to α-diketones in the presence of a chiral perhydro-1,3-benzoxazine ligand has been described (Scheme 5).

Scheme 5
(Org. Lett. 2017, 19, 7, 1516–1519)
If you are going interested in the science we are investigating and would like to join our group, then please contact us at jmandres@qo.uva.es